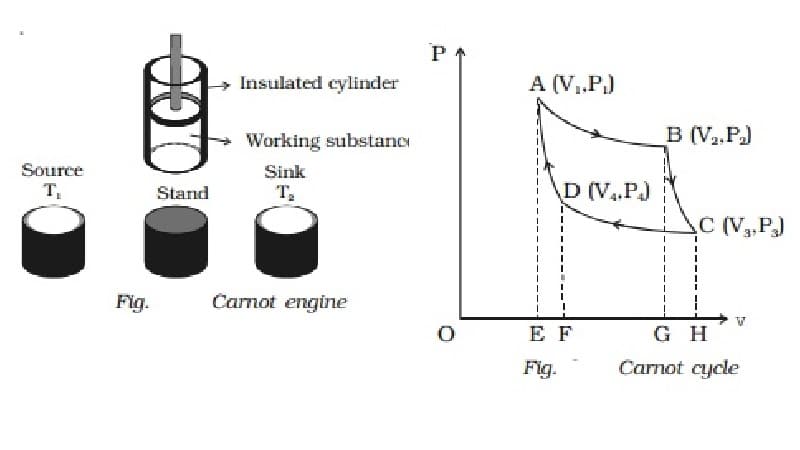
A Carnot engine is a device that converts thermal energy into mechanical energy. In 1824, Carnot created a Carnot cycle for a heat engine. The machine used to perform this cycle is called a complete operation, complete heat engine or Carnot heat engine.
All thermodynamic systems exist in a certain state.
A thermodynamic cycle occurs when the system goes through a number of different situations and eventually returns to its original form. When going through the Carnot cycle, the system can work in the environment and thus act as a Carnot heat engine.
The function of this engine is to transfer energy from a hot to a cold place and convert some of the power into work. You can also reverse the loop. The system can be exposed to external forces, transferring heat from the cold body to the warm body, which can act as a refrigerator pump instead of a heat engine.
Components of Carnot engine
(1) Source
This is a hot body that is kept constant at T1. It has unlimited heat capacity. Any heat can be extracted from it at a constant T1 temperature (that is, even if some heat is removed, its temperature remains constant.
(2) Sink
It is a cold body that is kept at a lower T2 temperature. Its heat capacity is not limited and the added heat does not increase its temperature. The sink has very temperature due to that heat flows from the hot body to the cold body. Therefore, the sink plays an important role in the working of the Carnot engine.
(3) Cylinder
The cylinder consists of a non-conductive wall and a conductive bottom. The ideal gas is used as a working element. The cylinder is equipped with a completely non-conductive and friction-free piston.
(4) Insulating stand
It is made of non-conductive material for adiabatic operations.
Carnot Engine Working Principle
Carnot’s principle applies only to rotating equipment such as heat engine, which states:
The efficiency of a non-reversible heat engine is always lower than that of a reversible heat engine operating between two identical tanks. The efficiency of all reversible heat engines operating between two identical tanks is the same.
The combustion chamber temperature must be increased to increase the thermal efficiency of the gas turbine. For example, turbine blades cannot withstand hot gases and cause premature fatigue.
Carnot’s Theorem
This theorem states that a motor that runs between two specific temperatures will not be more efficient than a reversible motor that runs between two identical temperatures, and regardless of the functional component, all reversible motors are between two and run at the same temperature. According to Carnot theorem, reversible motors always have higher efficiency than non-reversible motors. The reciprocating heat engine runs in reverse cycle and acts as a heat pump (or coolant).
Efficiency of the Carnot cycle
The Carnot cycle is reversible and indicates a high level of engine cycle efficiency. The actual cycle of the device is irreversible, so when operating at the same temperature, its efficiency is substantially lower than that of Carnot. One of the factors determining performance is the addition and removal of working fluid to the cycle. The Carnot cycle reaches its maximum efficiency because all the heat is added to the working fluid at the maximum temperature.
The rotation of the Carnot engine involves the following steps:
- Reversible isothermal expansion of a gas at “hot” temperature.
- Isentropic (reversible adiabatic) expansion of a gas.
- Reversible isothermal gas compression at “cold” temperature.
Disadvantages of Carnot Engine
It is impossible to make such engine. This is because no engine can deliver 100% output as input without any lose of energy.